Have you ever ridden on a balloon?
- Electron Configuration For Noble Gases
- What Are The Noble Gases
- Noble Gases Valence Electrons
- Noble Gases Valence Electron Configuration
Name: Argon Symbol: Ar Atomic Number: 18 Atomic Mass: 39.9 Number of Protons/Electrons: 18 Number of Neutrons: 22 Classification: Noble Gases Discovery: 1894 Discoverer: Sir William Ramsay. All the noble gases, except for helium, have 8 valence electrons. Helium has 2 valence electrons. The valence electrons participate in chemical bonding processes and are present in the outermost. The Noble Gases do not have an ionic radius. This is because they don't form ions. Ions are charged particles, and atoms become charged particles when they gain or lose electrons. Atoms only gain or lose electrons in an attempt to establish an octet, or 8 valence electrons. Octets are particularly stable electron arrangements. In this video we're going to be talking about how you can write electron configurations using noble gas notation and to be more specific in this video we're also going to be focusing on examples using main group elements so we're doing that because the transition metals right here and the lanthanides are a little bit more complicated so we won't be doing that in this particular video so the.
Many tourist spots offer balloon rides in order for people to see the beauty of a place from above. A balloon contains a noble gas called helium. Formerly, balloons contained hydrogen but hydrogen is very flammable and dangerous when uncontrolled. Therefore, people shifted to helium, which is safer. Helium is safe because it has the properties of the noble gases.
What is the history of noble gases?
People once believed that noble gases couldn’t chemically react at all. For this reason, they were called inert gases. They were also clustered under Group 0 in the old periodic table because scientists believed that the gases have zero valence electrons in their outer shell. This was later proven to be untrue when some noble gas compounds were discovered.
What are noble gases?
The gases are elements, which share similar properties. These properties include being monoatomic, colorless, odorless, being able to conduct electricity, and having low chemical reactivity. Noble gases include Helium, Neon, Argon, Krypton, Xenon and Radon. These are all found in Group 18, in the rightmost column of the periodic table. If you look at the periodic table, you will notice that these elements are the only ones, which do not have a charge. Helium has the lowest molecular weight while Radon is the heaviest.
Helium (He) is a component of natural gas. Argon (Ar), Neon (Ne), Xenon (Xe) and Krypton (Kr) are present in the air and can be isolated through liquefaction (the process of changing a gas into liquid through condensation) and fractional distillation (the separation of a mixture into its component parts by their boiling points.) Radon (Ra) is emitted as a result of the radioactive decay of radium compounds.
What causes the low chemical reactivity of noble gases?
Remember that chemical reactions occur because atoms have “valence” electrons, which are electrons in their outer shell. When the outer shell is “unfilled” or the required number of electrons is not yet complete, the atom is more reactive. Noble gases have a full outer shell, meaning that they have complete electrons in their outer shell. This complete number varies. For instance, the outer shell of Helium has 2 valence electrons while the outer shell of Xenon has 8 electrons. Nowadays, there remains to be a few noble gases because of the inherent low chemical reactivity of these said gases.
What are the applications of noble gases?
Because of their properties, noble gases have many important applications. They are widely used in recreation, medicine and industries. They are mainly used in conditions that require a stable and safe element.
For instance, deep-sea divers use a Helium-Oxygen gas combination in their tanks. When the diver goes beyond a depth of 55 meters (180 feet), there is a risk of Oxygen toxemia and Nitrogen narcosis. At high pressures as experienced in deep sea diving, gases like Oxygen and Nitrogen can be directly absorbed by the blood and by the tissues. Nitrogen narcosis is called such because at high pressures, nitrogen exerts an anaesthetic effect. With narcosis, the diver becomes sleepy and unable to move, which can lead to severe consequences underwater.
Helium is important because it has low solubility. It does not dissolve well in fluids and in lipids, which are components of your cell membranes. Therefore, only a small amount of Helium enters the cell membrane. Helium is a component of breathing mixtures like trimix and heliox. The use of Helium for breathing gas also reduces the risk for “the bends” or decompression sickness. This condition occurs when the diver comes back to the surface rapidly, leading to the formation of gas bubbles from formerly dissolved gas in the blood. With Helium, there is lesser amount of the dissolved gas, which helps the diver avoid the bends. Argon, on the other hand, is considered the best gas for the inflation of dry-suits used by scuba divers.

The boiling and melting points of noble gases are relatively low. For instance, Helium has a boiling and melting point of -268.95 degrees Celsius (-452.11 degrees Fahrenheit or 4.2 Kelvin). Liquid Neon has a refrigerating capacity, which is 40 times that of liquid helium and 3 times that of liquid hydrogen. Therefore, noble gases are very good refrigerants.
Liquid Helium is also used for superconducting magnets. These magnets are very important in physics and medicine. When a doctor suspects that a person’s brain has been damaged, he might request for Magnetic Resonance Imaging (MRI). MRI allows the doctor to “see” the brain, without operating on the patient.
Aren’t noble gases fascinating? Who knows, you might even be the next one to discover a rare noble gas compound! Get ready for your great scientific discoveries by learning more about Chemistry with our Introduction to Chemistry eBook.
Refresher: The periodic table is organized into groups - where each column comprises a group. All of the elements in a group share the same number of valence electrons: electrons in their outermost shell. Elements in the same group typically have similar chemical properties as a result of their similar electronic configuration. Elements in the periodic table can be described as metals, metalloids, and nonmetals
FAQs
1. What are the noble gases?
The noble gases make up group 18 of the periodic table. They are chemically inert, colorless, odorless nonmetal elements with a full shell of valence electrons.
2. Why are noble gases unreactive?
Noble gases are unreactive due to their full valence shells. Because they are already in the most stable electronic configuration, they do not easily gain or lose electrons.
3. How many valence electrons do noble gases have?
Noble gases have a full valence shell of 8 electrons.
4. Why are noble gases stable?
Noble gases are highly stable because they of their electronic configuration. They have a full outer shell of electrons, making them already in their most stable state. They do not seek to lose or gain electrons, meaning they do not easily react with other elements. This makes them very stable.
Electron Configuration For Noble Gases

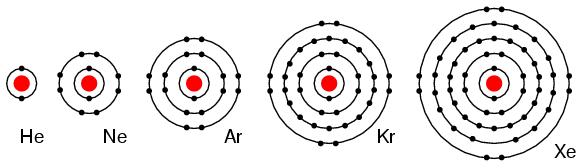
Properties of the Noble Gases
Group 18 of the periodic table contains the noble gases. These are the elements in the 18th column of the periodic table, at the far right. They are all nonmetals, and are found in their standard state as monatomic gases. Noble gases are relatively inert and nonreactive due to their full outer shell of electrons.
What Are The Noble Gases
Noble gases were discovered in 1895, when Sir William Ramsay and Lord Rayleigh isolated an unreactive chemical substance that was heavier than nitrogen from the air. This was the first element discovered of the group that came to be known as the noble gases.
Noble gases are a group of nonmetals in group 18 that are often described as chemically inert - they are colorless, odorless and highly unreactive. This group includes helium, neon, argon, krypton, xenon, radon, and the synthetic element oganesson. Noble gases are highly unreactive due to their full outer shell of electrons. Having a full valence shell of 8 electrons means that the atom is already in its most stable state, and thus, there is no need for the atom to lose or gain electrons.
The noble gases have some of the highest ionisation energies in the periodic table due to their stable structure, but some reactions (although few) do occur. For example, xenon, a noble gas, can combine with one or more fluorine atoms to form various xenon fluorides. These are highly stable compounds with more than 8 electrons in their outer shells. This reaction is possible because Xenon has a unique electron configuration which allows it to accept more than 8 electrons in its outermost shell.
Noble Gases Valence Electrons
As you progress down the periodic table, not much changes about the chemical properties of the noble gases. However, they do decrease in abundance - with helium, at the top of the group being the most plentiful noble gas.
Related Lessons
Noble Gases Valence Electron Configuration
