- Relative Atomic Mass Of Sodium
- Relative Atomic Mass Of Carbon
- Relative Atomic Mass Calculator
- Relative Atomic Mass Definition
Relative Atomic Mass Of Sodium
Relative atomic mass accounts for the mass and relative abundance of different isotopes of an element Why is the word 'relative' used for mass the weighted mean mass of all the naturally occuring isotopes of an element are relative to 1/12 the mass of a carbon-12 atom. Relative molecular mass definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. The relative atomic mass of an element is defined as the weight in grams of the number of atoms of the element contained in 12.00 g of carbon-12. The relative atomic mass of Copper is therefore (70 / 100 x 63) + (30 / 100 x 65) = 63.6 There is no unit as it is a relative value. Molecular M ass (M r ) is the sum of all the relative atomic masses for all the atoms in a given formula.
Click to see full answer
Relative Atomic Mass Of Carbon

Relative Atomic Mass Calculator
Simply so, why atomic mass is also called relative atomic mass?
Relative Atomic Mass Definition
Atomic mass is also called Relative Atomic Mass as it is calculated as the no. of times an atom of an element is heavier than 1/12 th the mass of an Carbon atom ( C-12 isotope ) .
Also Know, which is the atomic mass? The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
Consequently, is atomic mass the same as relative atomic mass?
Relative atomic mass is the ratio of the average mass of one atom of an element to one twelfth of the mass of an atom of carbon-12. Atomic mass is just the mass of atoms in an element BUT they are not necessarily the same mass so we get Chlorine as 35.453 u ± 0.002 u!

What is the difference between relative atomic mass and average atomic mass?
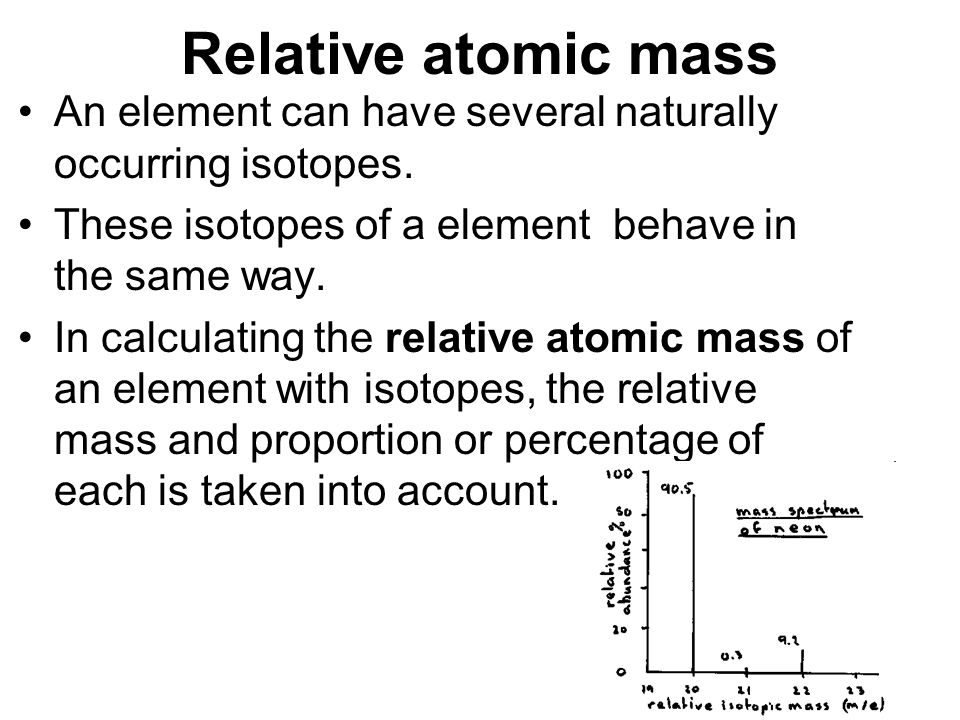
Relative and average atomic mass both describe properties of an element related to its different isotopes. However, relative atomic mass is a standardized number that's assumed to be correct under most circumstances, while average atomic mass is only true for a specific sample.
